CALCIUM LACTATE PENTAHYDRATE
PRODUCT IDENTIFICATION
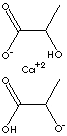
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
white powder
GENERAL DESCRIPTION & APPLICATIONS
- Ammonium Lactate (NH4C3H5O3, CAS RN: 515-98-0): clear to yellow, syrupy liquid used in in electroplating, in finishing leather and as humectant for food, pharmaceutical, and cosmetics.
- Butyl Lactate (CH3CHOHCOOC4H9, CAS RN:138-22-7): a clear liquid: nontoxic, miscible with many solvents; used as a solvent for varnish, lacquers, resins and gums, used in making paints, inks, dry cleaning fluid, flavoring and as a chemical intermediate.
- Calcium Lactate Pentahydrate [Ca(C3H5O3)2·5H2O, CAS RN: 814-80-2] : white crystals; soluble in water; used as a calcium source; administered orally in the treatment of calcium deficiency; as a blood coagulant.
- Ethyl Lactate (CH3CHOHCOOC2H5, CAS RN: 97-64-3): clear liquid with mild odur; boiling point 154 C; miscible with alcohols, ketones, esters, and hydrocarbons as well as with water; used in pharmaceutical preparations, feed additive, as a flavoring ( odor description: sweet butter, coconut, fruity, creamy dairy, butterscotch) and as a solvent for cellulose compounds such as nitrocellulose, cellulose acetate, and cellulose ethers.
- Magnesium Lactate Trihydrate [Mg(C3H5O3)2·3H2O, CAS RN: 18917-93-6 ]: white crystals with bitter taste; soluble in water, slightly soluble in alcohol; used in medicine and as an electrolyte replenisher.
- Manganese Lactate Trihydrate [Mn(C3H5O3)2·3H2O]: pale red crystals; insoluble in water and alcohol; used in medicine.
- Mercuric Lactate [Hg(C3H5O3)2]: poisonous white powder that decomposes when heated; soluble in water; used in medicine.
- Methyl Lactate (CH3CHCHCOOCH3): clear liquid with mild odur; boiling point 145 C; miscible with alcohols, ketones, esters, and hydrocarbons as well as with water; used in pharmaceutical preparations, feed additive, as a flavoring and as a solvent for cellulose compounds such as nitrocellulose, cellulose acetate, and cellulose ethers.
- Sodium Lactate (CH3CHOHCOONa, CAS RN: 72-17-3) clear to yellow, hygroscopic syrupy liquid; soluble in water; melting point 17 C; used in medicine, in antifreeze, and hygroscopic agent and as a corrosion inhibitor.
- Zinc Lactate (Zn(C3H5O3)2·2H2O, CAS RN: 16039-53-5): white crystals; used as an additive in toothpaste and food; preparation of drugs.
APPEARANCE
white powder
ASSAY
98.0-101.0%
CALCIUM ASSAY
13.4-14.5%
LOSS ON DRYING
REDUCING SUGARS
Pass Test
HEAVY METALS
20ppm max
IRON
40ppm max
LEAD
1ppm max
ARSENIC
1ppm max
SULPHATE
400ppm max
CHLORIDE
80ppm max
ALKALI SALTS
1.0% max
MESOPHILIC BACTERIA
1000/g
MOULD
100/g
YEAST
100/g
OTHER INFORMATION